Saw this on the generation of hydrogen this morning. From phys.org

Natural clay magadiite containing silicon (Si) was heated in a sealed vessel in a water-based solution containing iron chloride (FeCl3) and nickel chloride (NiCl2) to create a metallic silicate made up of nickel (Ni), iron (Fe) and Si. The metallic silicate was then reduced by adding electrons to metallic silicate atoms with magnesium, salt and heat to create the more organized intermetallic silicide (ferric-nickel silicide) structure. The graph illustrates the lower voltage required for the ferric-nickel silicide (FeNiSi) alloy electrocatalyst to produce hydrogen and oxygen gas compared to NiSi and FeSi alloys. Credit: Nano Research Energy, Tsinghua University Press
________________________________________________________________________________________________________________________
A new electrocatalyst made of nickel (Ni), iron (Fe) and silicon (Si) that decreases the amount of energy required to synthesize H2 from water has been manufactured in a simple and cost-effective way, increasing the practicality of H2 as a clean and renewable energy of the future.
Hydrogen is a highly combustible gas that can help the world achieve its clean energy goals if manufactured in an environmentally responsible way. The primary hurdle to creating hydrogen gas from water is the large amount of energy required for the electrolysis of water, or splitting water molecules into hydrogen gas (H2) and oxygen (O2).
Most H2 produced today is derived from fossil fuels, which contributes to global warming. Manufacturing H2 from water through the hydrogen evolution reaction (HER) requires the use of a catalyst, or agent that lowers the amount of energy required for a chemical reaction. Until recently, these catalysts were made up of rare earth metals, like platinum, reducing the cost-efficiency and practicality of clean hydrogen production.
A group of material scientists from Dalian University of Technology in Dalian, China manufactured an electrocatalyst, or a catalyst that uses electricity, using inexpensive materials and methods to effectively decrease the energy required to generate clean H2 from water. Importantly, the ferric-nickel silicide (FeNiSi) alloy, or mixture, also reduces the energy required to generate O2 from water, making the catalyst bifunctional.
more ...
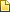 = new reply since forum marked as read
= new reply since forum marked as read
