Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
Editorials & Other Articles
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Science
Related: About this forumLithium Concentrations in Groundwater Used as Drinking Water for the Conterminous United States.
The paper to which I'll refer in this post is this one: Estimating Lithium Concentrations in Groundwater Used as Drinking Water for the Conterminous United States Melissa A. Lombard, Eric E. Brown, Daniel M. Saftner, Monica M. Arienzo, Esme Fuller-Thomson, Craig J. Brown, and Joseph D. Ayotte Environmental Science & Technology 2024 58 (2), 1255-1264
The article is open access for the public to read, nevertheless I'll offer a brief excerpt for convenience.
From the introduction of the paper:
Lithium (Li) is a naturally occurring alkali metal found in minerals and in groundwater and surface water as a monovalent cation. Li concentrations in drinking-water supplies are not currently regulated in the United States; therefore, its occurrence has not been commonly measured. Li is used as a medication to treat bipolar disorder and depression, (1) and clinical doses typically range between 600 and 1800 mg per day, which is 2–3 orders of magnitude greater than typical drinking-water concentrations (less than 0.030 mg/L). (2) Studies have linked the low-level occurrence (0.1–219 μg/L) of Li in drinking water to positive human-health outcomes such as reduced suicide mortality (3−7) and other mental-health benefits (2,8−11) in addition to potential negative outcomes such as autism (12) and thyroid hormone levels. (13,14) Further, side effects impacting renal, neurological, dermal, cardiovascular, and endocrine systems can occur due to Li used clinically, especially at higher doses (2.74–4.2 mg Li/kg of body weight per day). (15) The U.S. Environmental Protection Agency (EPA) established a provisional reference dose (p-RfD) of 2 μg of Li per kilogram of body weight per day and reports that confidence in this value is low to medium because of a lack of dose–response information at subclinical concentrations. (15) The U.S. Geological Survey developed a nonenforceable health-based screening level of 10 μg/L for Li in drinking water based on the p-RfD. (16) Li is included in the most recent EPA list of unregulated contaminants to be monitored by public water systems as part of the fifth Unregulated Contaminant Monitoring Rule (UCMR5). This rule requires public water suppliers to measure Li concentrations in drinking water starting in 2023 to provide nationally distributed data on its occurrence. (17) The inclusion of Li in UCMR5 indicates the potential for future regulations in public drinking-water supply utilities.]
A graphic from the paper:
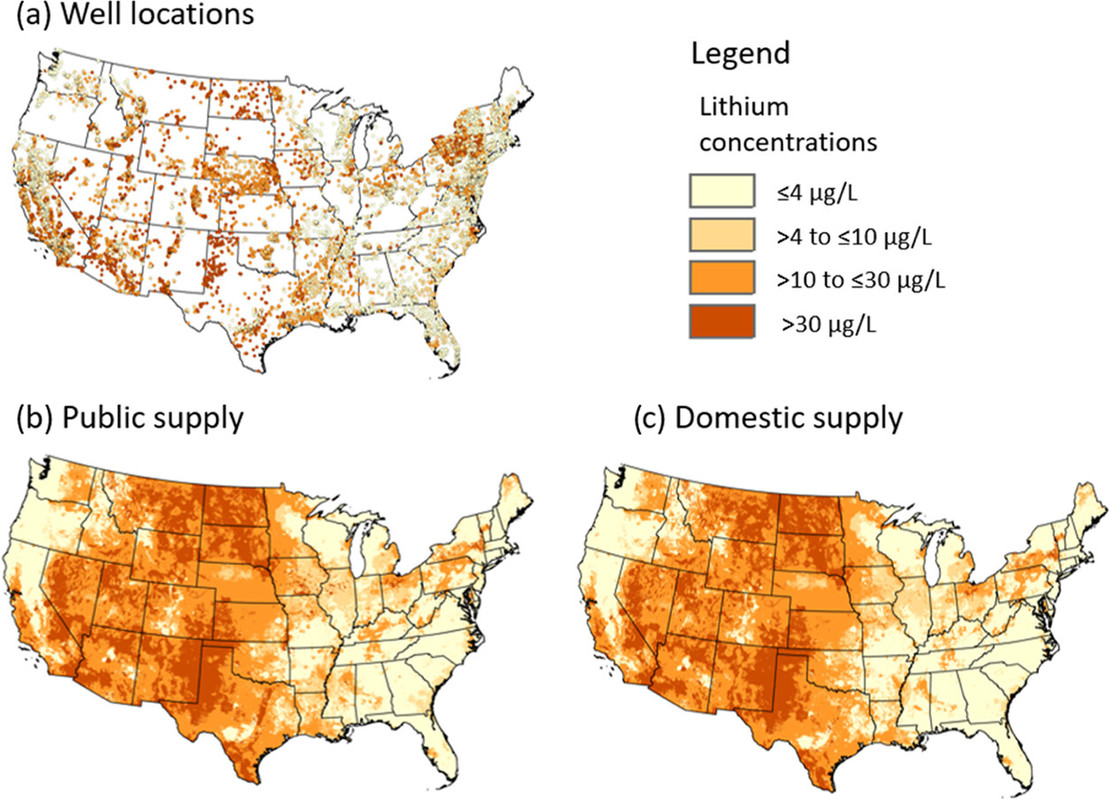
The caption:
Figure 1. Lithium concentrations in groundwater (a) at well locations, (b) modeled for public-supply well depths, and (c) modeled for private-supply well depths.
No comment, other than that figure 6 in the paper, if one opens it, is interesting.
InfoView thread info, including edit history
TrashPut this thread in your Trash Can (My DU » Trash Can)
BookmarkAdd this thread to your Bookmarks (My DU » Bookmarks)
3 replies, 1364 views
ShareGet links to this post and/or share on social media
AlertAlert this post for a rule violation
PowersThere are no powers you can use on this post
EditCannot edit other people's posts
ReplyReply to this post
EditCannot edit other people's posts
Rec (7)
ReplyReply to this post
3 replies
 = new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
= new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
Lithium Concentrations in Groundwater Used as Drinking Water for the Conterminous United States. (Original Post)
NNadir
Jan 2024
OP
Quite a lithium shortage here in the Southeast -- perhaps that explains some things ! :)
eppur_se_muova
Jan 2024
#2
Response to NNadir (Original post)
Name removed Message auto-removed
eppur_se_muova
(37,397 posts)2. Quite a lithium shortage here in the Southeast -- perhaps that explains some things ! :)
Ironically, I bought my first car in Lithia Springs, GA. :^/

erronis
(16,827 posts)3. Fascinating. Wikipedia article
https://en.wikipedia.org/wiki/7_Up
7 Up was created by Charles Leiper Grigg, who launched his St. Louis–based company The Howdy Corporation in 1920.[1] Grigg came up with the formula for a lemon-lime soft drink in 1929. The product, originally named "Bib-Label Lithiated Lemon-Lime Soda", was launched two weeks before the Wall Street Crash of 1929.[2] It contained lithium citrate, a mood-stabilizing drug, until 1948.[3][4] It was one of a number of patent medicine products popular in the late-19th and early-20th centuries. Its name was later shortened to "7 Up Lithiated Lemon Soda" before being further shortened to just "7 Up" by 1936.[5]
The origin of the revised name is unclear.[6] Britvic claims that the name comes from the seven main ingredients in the drink,[a][8] while others have claimed that the number was a coded reference to the lithium contained in the original recipe, which has an atomic mass of 7.[9] Britvic also claims that the name alluded to 7 Up seven-ounce bottles when Coca-Cola and most other soft drinks were bottled in six-ounce bottles.
The origin of the revised name is unclear.[6] Britvic claims that the name comes from the seven main ingredients in the drink,[a][8] while others have claimed that the number was a coded reference to the lithium contained in the original recipe, which has an atomic mass of 7.[9] Britvic also claims that the name alluded to 7 Up seven-ounce bottles when Coca-Cola and most other soft drinks were bottled in six-ounce bottles.