Science
Related: About this forumOh Oh. Pollution Abatement Catalyst Generates the Ozone Depleting Greenhouse Gas N2O.
The paper to which I'll refer in this post is this one: Potential Risk of Significant N2O Emission without Changing NOx Conversion on Commercial V2O5/TiO2 Catalyst under Working Conditions Jiaying Xing, Qitong Xue, Jianjun Chen, Jinxing Mi, Xiaoping Chen, Jianqiang Shi, Zhiming Liu, and Junhua Li Environmental Science & Technology 2023 57 (51), 21866-21875.
There is always the potential that in trying to fix one problem, one can create a worse problem. Anyone who is familiar with my writing we recognize for instance that I contend that so called "renewable energy" has made climate change worse at a cost of trillions of dollars. The observation that the accumulations of the dangerous fossil fuel waste carbon dioxide has accelerated in the planetary atmosphere during a period where trillions of dollars have been spent on solar and wind energy is unambiguous data, but the causality, whether the redundancies required by solar and wind and their inherent dependence on access to dangerous fossil fuels to address their intrinsic lack of reliability, is subject to argument, poor arguments in my opinion, but argument all the same.
The lie is often told that so called "renewable energy" has led to the decline in the use of coal, but this is also nonsense. The data showing this to be nonsense is available in the most recent World Energy Outlook, the 2023 edition:
The numbers are here: 2023 World Energy Outlook published by the International Energy Agency (IEA), Table A.1a on Page 264.
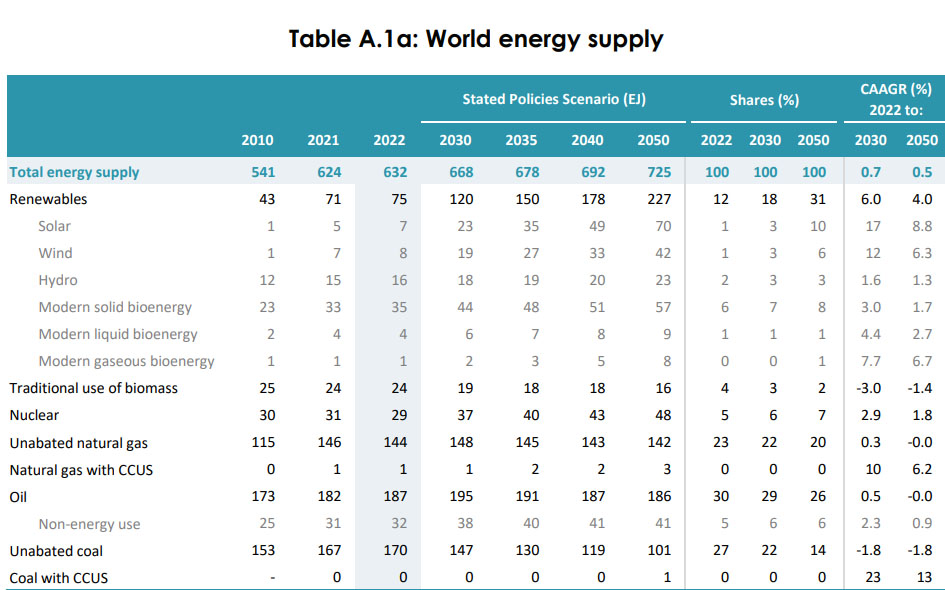
There is, of course, soothsaying that coal use will decline; it's been going on for years, albeit not so much involved with buying charms from a lady with a crystal ball at a stand on the Jersey Shore to fix your rotten love life, but about as accurate as the predictions these charlatans make about how your ex-girlfriend or ex-boyfriend will come back to you when he or she tires of his or her new extremely good looking and extremely rich new lover against whom you had zero chance ever of competing.
(I was an adolescent once, well into my thirties, a source of some amusement.)
Anyway...
On this planet, we are using more coal than we have ever used, more gas than we ever used, and more oil than we ever used, although some countries, including my country, export pollution by importing manufactured goods rather than making them here and generating the resultant pollution here, and then cry loudly that they are "going green."
That's a fact.
As for the value of so called "renewable energy" for fighting climate change, there is none, which is hardly surprising because this reactionary scheme for returning to the 18th and early 19th century dependence of energy supplies on the weather was never conceived to address climate change. It was promoted to attack nuclear energy, thus demonstrating contempt for worrying about climate change. It was a reactionary scheme to attack a progressive form of energy.
Anyway...
One of the ways that the coal industry pretends to be "clean" - everything everywhere calls itself clean and green, even coal - is by catalytic treatment of flue gas to remove major pollutants. This is difficult to do for heavy metal pollution, coal ash contains mercury, lead, and (gasp) uranium but various approaches have been generated for the light element oxides, in particular sulfur oxides and nitrogen oxides. For the latter, an approach known as "SCR" (selective catalytic reduction) is often used - it is also used in certain diesel vehicles - where a reduced form of nitrogen, often urea but ammonia will work, reacts with oxidized nitrogen, often called NOx to give neutral nitrogen gas.
One nitrogen oxide, in many ways the worst one, the generation of which our food supplies depend, is nitrous oxide, aka "laughing gas," N2O, because it is relatively stable, and is both an ozone depleting agent and a greenhouse gas, the third most important after carbon dioxide and methane. Concentrations of this gas are rising.
It appears, if this paper is accurate, that the pollution treatment in the SCR framework with vanadium oxide catalysts supported on titanium dioxide - the commercial and deployed technology - does not always produce nitrogen gas, but also generates N2O, as suggested by the paper's title. This occurs when chromium, another heavy metal found in coal ash - chromium in the +6 oxidation state is a powerful carcinogen - and flue gas deposits on the vanadium catalyst. In this case, a case of mild catalytic "poisoning" instead of N2 gas, partially reduced N2O is formed.
I can't spend a lot of time discussing this paper in detail, but here's a few excerpts:
N2O formation in the NH3–SCR process by vanadium-based catalysts is determined by several critical factors, such as reaction pathway, rate-determining step, etc. It is generally accepted that N atoms in N2O originate from one NH3 molecule and one NO molecule, while NO, gaseous O2, and catalyst oxygen all function as the oxygen source for N2O. (9−14) However, a dispute existed for the nonselective formation of N2O with two common pathways: Krishna et al. (15−17) suggest that N2O formation is attributed to the decomposition of ammonium nitrate species that is reacted by NH3and NOx at low temperatures (less than 300 °C). When the temperature rises to 300 °C, NH3 oxidation by O2 dominates the N2O generation. Another alternative point is proposed that N2O forms by the reaction between surface NHads and/or NH2,ads species with adsorbed NO. (18−20) However, a consensus has not been reached on the N2O formation mechanism during the NH3–SCR process and needs further exploration.
Limited by the optimal operating temperature of commercial vanadium-based catalysts (300∼400 °C), the SCR unit is normally arranged upstream of the dust collector and desulfurizer in industrial applications. This implies that the catalyst can be easily abrased by fly ash. Besides, the denitration efficiency can be greatly affected by the alkali (earth) metal, heavy metals, steam, and sulfur dioxides in complex flue gas. (21−23) Chromium (Cr) is a typical heavy metal existing in complex flue gases which can cause an inevitable impact on NH3–SCR performance. Cr concentration in industrial materials is relatively higher than some other heavy metals, such as mercury, arsenic, etc. For example, Cr concentration in raw coal is in the range of 10∼100 μg/g, (24−26) which is higher than those of arsenic (0.5∼80 μg/g) (27) and mercury (about 1 μg/g). (28) During coal combustion, a part of Cr evaporates into flue gas in either gaseous form or solid phase in fly ash. Then, the enriched Cr in fine particle or gaseous Cr will go into the SCR unit, resulting in unneglected effect on catalysts. (29) Li et al. (29) investigated Cr impact on γ-Fe2O3 catalyst and concluded that NOx conversion is promoted by Cr deposition at low temperatures (100∼300 °C), while it shows a negative impact at higher temperatures (300∼400 °C). In addition to coal-fired power plants, Cr concentration in the flue gas of cement industry and steel industry can reach as high as 65 and 70 μg/Nm3. (30)...
A figure from the text:
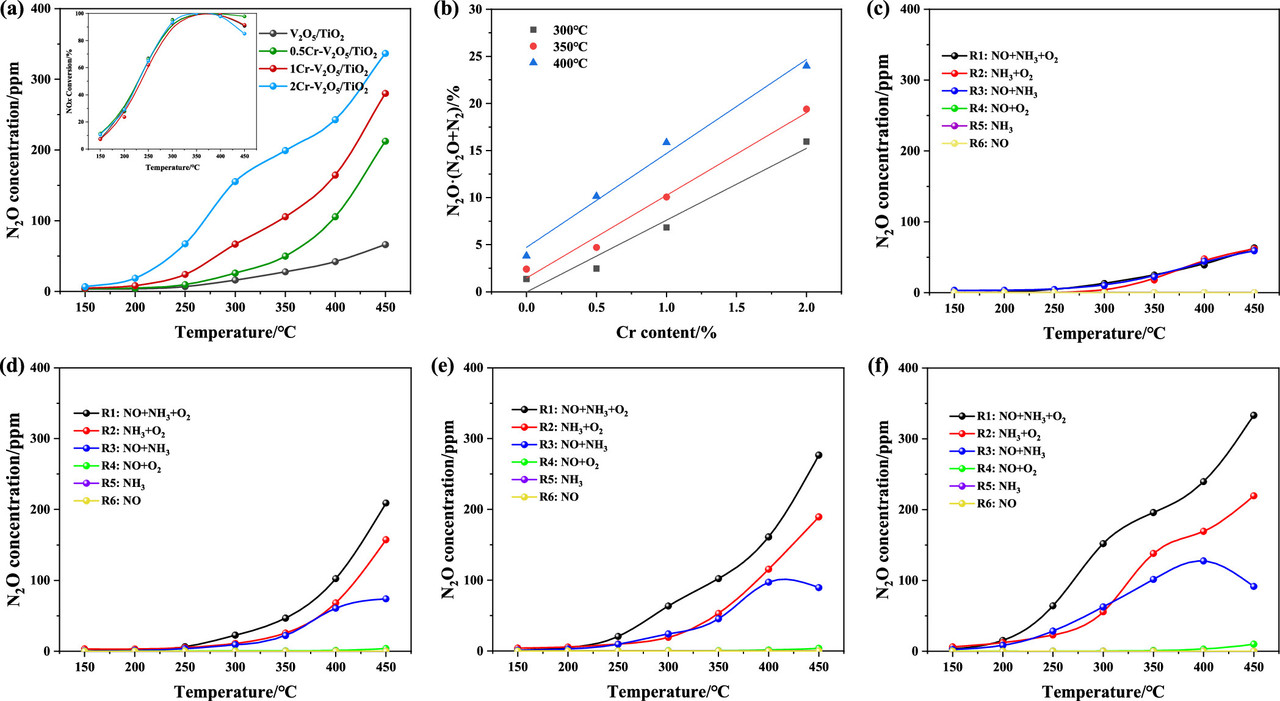
The caption:
From the conclusion:
Like George Orwell used to say, "doubleplusungood."
Have a nice day tomorrow.