Science
Related: About this forumAn organic catalyst for PFAS degradation with hydrated electrons and OH radicals.
The paper to which I'll briefly refer is this one: New Indole Derivative Heterogeneous System for the Synergistic Reduction and Oxidation of Various Per-/Polyfluoroalkyl Substances: Insights into the Degradation/Defluorination Mechanism Zhe Wang, Xin Jin, Ran Hong, Xinhao Wang, Zhanghao Chen, Guandao Gao, Huan He, Jinyong Liu, and Cheng Gu Environmental Science & Technology 2023 57 (50), 21459-21469
In a recent post earlier this evening, I referred to the possible carcinogenic (or more properly metasisogenic) properties of the "forever chemicals," PFAS, perfluoroalkyl substances (also polyfluoroalkylated substances).
Later in the same issue of Environmental Science & Technology the paper currently under discussion occurred.
In other posts in this space, I have noted that the strong carbon fluorine bond generally requires far UV energy, around 220 nm, to break. This paper discusses a catalyst that allows slightly longer wavelength UV, more accessible UV, at 254 nm to break the bond via catalysis.
From the paper:
The C–F bond has an extremely high bond energy of 100–120 kcal/mol. (18,19) Thereby, traditional wastewater treatment technologies, such as advanced oxidation processes (AOPs) and microbial degradation, are inefficient in degrading PFAS. (21−24) In recent years, advanced reduction technologies that rely on hydrated electrons (eaq–) have shown high efficiency in degrading and defluorinating PFAS. (25) This is due to the strong reductive nature of epaq– (−2.87 V, vs SHE), which can directly cleave the C–F bond. (26) Several compounds, such as sulfites, iodide ions, nitrilotriacetic acid (NTA), indole derivatives, etc., have been applied asepaq– precursors under ultraviolet light (UV, 254 nm) conditions. (19,27−29) To further improve the epaq– utilization efficiency, we synthesized a new indole compound (di-indole hexadecyl ammonium, DIHA), which is decorated with a hexadecane chain and a tertiary amine center (Scheme 1). (30) It can form stable nanospheres (100–200 nm) in water via supramolecular assembly. These DIHA nanospheres can provide long-range electrostatic, hydrophobic, and van der Waals (vdW) interactions to attract and adsorb PFOA and PFOS. (30) Under UV (254 nm) irradiation, eaq– can be generated from DIHA molecules, which can then degrade and defluorinate PFOA and PFOS highly efficiently. (30) Compared to sulfite or other indole compound-sourced epaq– systems, the newly developed DIHA system exhibits a 1–2 order of magnitude higher eaq– utilization efficiency. (30) However, the underlying mechanism to ensure its great performance has not yet been fully explained, and its applicability to degrade different structured PFASs needs further examination...
Some pictures from the text:
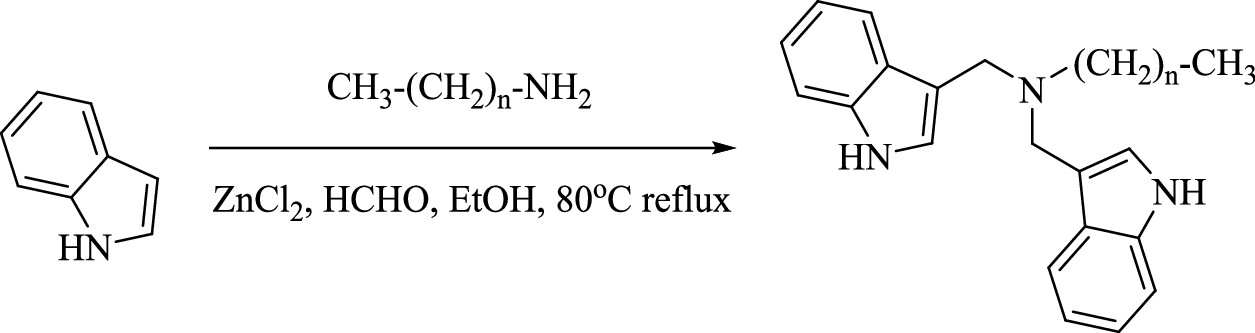
The caption:
A measure of the bond energies of these recalcitrant carbon-fluorine bonds

The caption:
A possible mechanism of degradation:
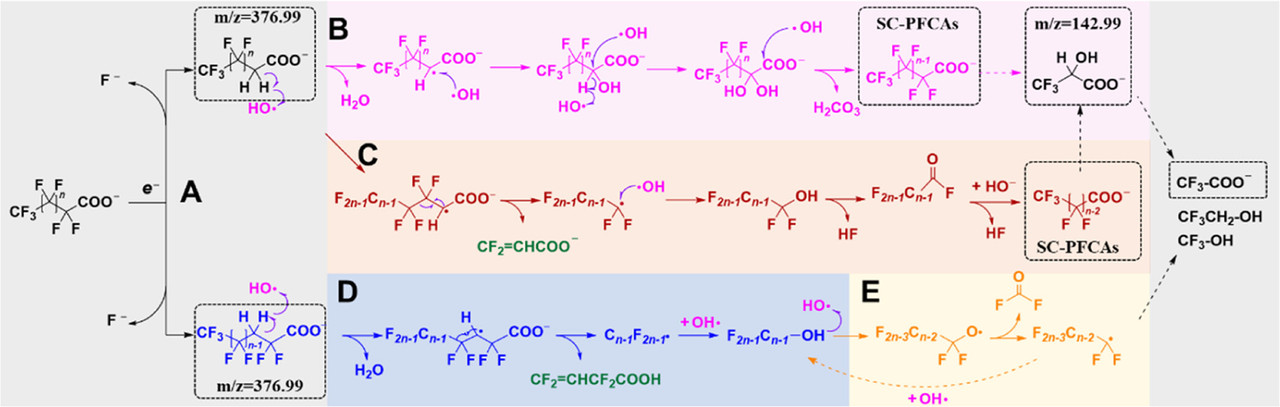
The caption:
aDetected products are marked in the dotted box.
From the conclusion:
Nice and interesting work.
I wish you a healthy and happy New Year.
eppur_se_muova
(40,701 posts)I hope they try another zinc (or other) salt as Lewis acid.
The radiation catalysis is impressive, however.