Science
Related: About this forumA Bacterial Protein Can Transport Heavy Highly Radioactive Actinides.
Last edited Thu Dec 21, 2023, 03:37 AM - Edit history (1)
The paper I'll discuss in this post is this one: Impact of a Biological Chelator, Lanmodulin, on Minor Actinide Aqueous Speciation and Transport in the Environment Gauthier J.-P. Deblonde, Keith Morrison, Joseph A. Mattocks, Joseph A. Cotruvo Jr., Mavrik Zavarin, and Annie B. Kersting Environmental Science & Technology 2023 57 (49), 20830-20843.
In this space, I previously discussed, at some length - considerable length actually - on a paper written by two of the authors of the paper just cited, Mavrik Zavarin and Annie B. Kersting.. Actually I covered a lot of papers in that post which was fascinating to write, but probably challenging and/or impossible to read.
The post is here: 828 Underground Nuclear Tests, Plutonium Migration in Nevada, Dunning, Kruger, Strawmen, and Tunnels.
I fessed up about it right out of the box about the desultory nature of that post, but irrespective of my carrying on about it (and indeed the current paper under discussion), Drs Kersting and Zavarin are outstanding, excellent scientists in my opinion, having had exposure to their work.
Here's what I wrote back then:
One of the papers I'll discuss in this post is this one: Plutonium Desorption from Nuclear Melt Glass-Derived Colloids and Implications for Migration at the Nevada National Security Site, USA (Claudia Joseph, Enrica Balboni, Teresa Baumer, Kerri Treinen, Annie B. Kersting, and Mavrik Zavarin Environmental Science & Technology 2019 53 (21), 12238-12246)
The reference to Dunning, Tunnels and Strawmen was a reference to the inspiration for the post was a moron of my acquaintance who felt it necessary to let me know that a tunnel with some old mildly radioactive rail cars had collapsed on the Hanford Nuclear Site and thus my advocacy of nuclear energy was unworthy. There was a lot of pissing and moaning and whining in follow up interactions with the insufferable idiot, and eventually I put the idiot on my ignore list where he/she/they/it/them happily resides to this day.
"All we are saying, is give peace a chance."
One of the things about antinukes, including "I'm not an antinuke" antinukes, is that they don't know shit from shinola when it comes to nuclear issues, although this doesn't stop them from commenting on them, usually in an insipid fashion.
By the way, your Government spent huge amounts of money, and generated many tons of dangerous fossil fuel waste - diesel exhaust mainly - to address the collapsed tunnel, which saved zero lives, because in reality zero lives were really at risk from radiation, although lives may be at risk from diesel exhaust, because the paranoia of people like the tiresome fool on my ignore list carries way more weight than it should or would in a rational world.
Anyway, to turn to the Dr. Kersting's and Dr. Zavarin's et al paper currently under discussion, it refers to the protein binding of two important (and in my view precious) actinide transuranium elements produced in nuclear power plants, curium and americium.
From the introduction:
Metalloproteins could affect the migration of certain radionuclides as is demonstrated here with lanmodulin and trivalent actinides.
Introduction
In recent years, there has been a renewed interest in using nuclear energy for electricity production (1) due to its low greenhouse gas emissions, small land footprint, and nonintermittent operation. There are currently 412 commercial nuclear fission reactors in operation around the world, (2) 58 are under construction, and several countries have plans to further expand or update their fleet of nuclear fission power plants. There are currently 93 reactors in operation in the United States, including a new 1100 megawatt reactor at Plant Vogtle (Georgia) that entered commercial operation on July 31st, 2023. While fission of uranium-only fuels or mixed uranium–plutonium oxide fuels currently represents one of the means of energy production with the lowest carbon and land footprint, it nonetheless produces long-lived, radioactive waste comprised of fission product, plutonium (Pu), and minor actinide (e.g., neptunium (Np), americium (Am), and curium (Cm)) isotopes that must be managed appropriately. A typical uranium-only civilian nuclear spent fuel, cooled for 5 years, still contains 13–18 kg lanthanide fission products, 10–13 kg Pu, 0.5–0.8 kg Np, ∼0.6 kg Am, and 0.03–0.12 kg Cm per metric ton of fuel. (3) For mixed uranium–plutonium oxide fuels, these numbers are 12–17 kg lanthanide fission products, ∼30 kg Pu, ∼2 kg Np, ∼2 kg Am, and ∼0.4 kg Cm per metric ton of fuel. The International Atomic Energy Agency estimates (4) that between 1954 and 2016, the equivalent of 390,000 t of U+Pu metal has been discharged from nuclear reactors worldwide. By the end of 2016, about 137,000 t had been reprocessed and 263,000 t remained in temporary storage. Disposal in deep underground repositories is largely seen as the safest option for the long-term management of spent fuels and nuclear waste, whether or not they have undergone reprocessing (e.g., PUREX process (5)). These repositories are meant to isolate the nuclear materials for thousands of years─the time scale required so that the radioactivity returns to a level similar to that of naturally radioactive ores. While the waste packages are designed to initially isolate the radioactive materials when they are the most radioactive, those engineered barriers will ultimately fail due to underground mechanical forces and natural alteration processes. In this context, contact between radionuclides and the underground natural environment (e.g., minerals, groundwater, metal chelators, as well as microorganisms) is expected to take place, and thus, studying the chemistry of radioelements under environmentally relevant conditions is critical to assess the long-term behavior of nuclear waste. (6−10)
My enormous respect for the authors aside, let me comment that I do not agree, in any fashion, with burying any components of used nuclear fuel in "deep underground repositories." I have convinced myself that all of the components of used nuclear fuel are valuable resources, more or less essential for human (and in fact, planetary) survival.
The figures for the actinide components of the fuel are worthy of consideration. The marvelous Vogtle reactors recently completed in Georgia will each be loaded with 82 (metric) tons of enriched uranium oxide. When this fuel is removed from the reactor after use, following the figures above, this suggests, for the upper limits, (first pass without initial plutonium (MOX) fuel) the fuel will contain a little over 1 ton of plutonium, 65 kg of neptunium, 50 kg of americium, and about 10 kg of curium. If we take, as an approximate figure, the fission of an actinide to be roughly equivalent to that of plutonium, 80 trillion Joules per kg, and the US EIA's figure for an average American annual energy demand, determined from the 100 Quads reported by the EIA (106 EJ) for a population 2022 of 333,000,000 million people, giving a per capita demand of 311 GJ/person, the energy content of the combined transuranium actinides in the Vogtle used nuclear fuel (after 3 years of operation) would be enough to provide all of the annual energy demand of around 320,000 people. Note too, that all of the uranium in the used nuclear fuel has the potential to be transmuted into transuranium nuclides.
If we assume that 95% of the 263,000 metric tons of used unprocessed nuclear fuel is unreacted uranium, which can be converted to plutonium and other transuranium nuclides, the energy content of used nuclear fuel is enough to supply all of the world's energy demands (at the 2022 figure of 634 EJ of energy) is enough to fuel the entire world for about 30 years.
Maybe we should think twice before burying the stuff.
Anyway.
It appears that certain ubitiquous bacteria, Methylorubrum extorquens generates a protein, lanmodulin, that has exceptional ability to complex actinides in the +3 oxidation state, having even more affinity for the actinides than the already high affinity for the related lanthanide congeners:
Lanmodulin is a relatively small protein, just 130 amino acids in length according to Uniprot, with the following sequence:
MAFRLSSAVLLAALVAAPAYAAPTTTTKVDIAAFDPDKDGTIDLKEALAAGSAAFDKLDPDKDGTLDAKELKGRVSEADLKKLDPDNDGTLDKKEYLAAVEAQFKAANPDNDGTIDARELASPAGSALVNLIR.
The metal binding sequences are shown here:
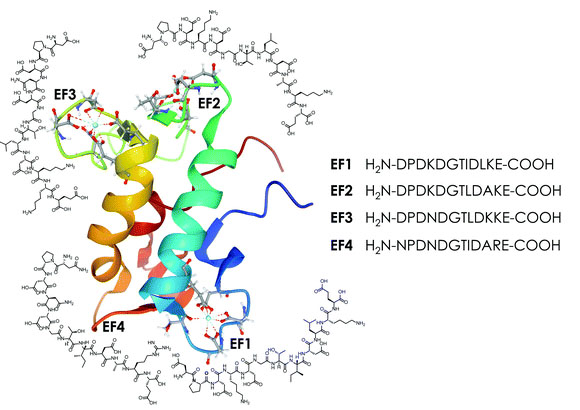
The caption:
Sophie M. Gutenthaler, Satoru Tsushima, Robin Steudtner, Manuel Gailer, Anja Hoffmann-Röder, Björn Drobot and Lena J. Daumann Lanmodulin peptides – unravelling the binding of the EF-Hand loop sequences stripped from the structural corset. Inorg. Chem. Front., 2022, 9, 4009-4021.
The authors consider that these organisms have the potential to solubilize the transplutonium actinides, in practice Am3+ and Cm3+ after breakdown of containment drums in deep disposal sites. (The solution to this "problem" is to not build deep disposal sites in the first place, but rather to put all of the components of used nuclear fuel to important uses.)
It is worth noting that there are a class of drugs known as ADC's, antibody drug conjugates, that are designed to carry highly radioactive atoms selectively to cancer cells. In this case, radiopharmaceuticals, a very special kind of ADC is prepared. Traditional ADCs carry a chemical payloads to the surface displays of cancer cells, where as their radioactive analogues carry radioactive atoms to the cancer cell surfaces, with a linker attached to a DOTA complex of a radioactive atom, often an isotope of actinium. The point is that if enough Am3+ and Cm3+ is carried in significant amounts, amounts high enough to matter, like cancer cells, the carrying cells are likely to be killed by the payload.
Again though, the point is not to bury actinides, but to praise them, treasure them, and put them to use.
An interesting application for this work would be to engineer immobilized organisms, perhaps mat forming bacteria to display lanmodulin on the the cell surface, and thus produce an effective filter or recovery system for valuable Am3+ and Cm3+.
Have a nice day tomorrow.
eppur_se_muova
(37,984 posts)
This is what I still think of when I think of the heavier actinides -- first isolation of macroscopic samples during the Manhattan Project. Two spaces of the Periodic Table were filled by *classified* wartime discoveries !
NNadir
(35,008 posts)...as a nuclear fuel, a very high neutron multiplicity.
The most commonly available isotope is 241Am, the decay product of 241Pu (t1/2 = 14.290 years).
It is definitely accumulating in used nuclear fuels, and, in fact, unused isolated reactor grade plutonium, like the significant stockpile in the UK.
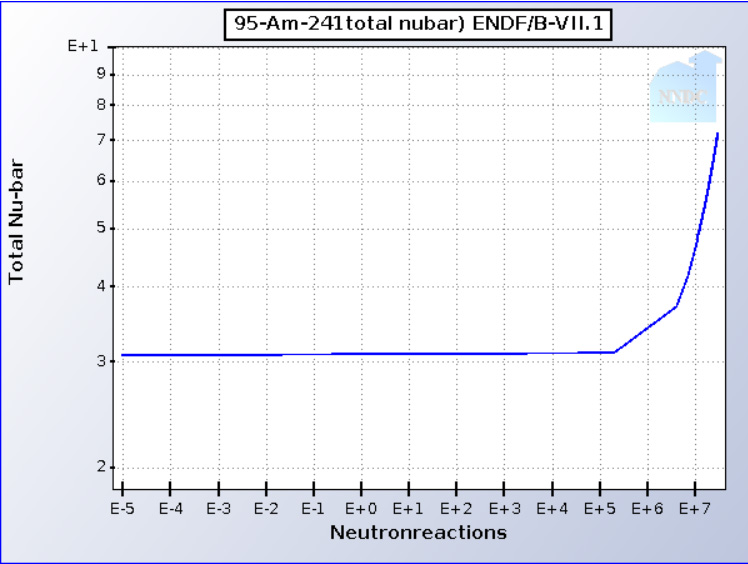
The neutron multiplicity (aka "nubar" ) is shown in this graph:
BNL Nuclear Data
241Am is not really fissionable in thermal reactors, as the capture to fission ratio is too high but it can reach a critical mass in the fast neutron spectrum. Critical masses of the three accessible Americium Isotopes. In cases where capture occurs, it creates the highly fissionable 242Am and 242mAm nuclear isomers, the latter of which can accumulate in considerable fractions, owing to its long half-life, (t1/2 = 141 years). It's multiplicity in the fast spectrum approaches 4.
It takes three neutron captures to generate 241Am. (Interestingly the precursor, 241Pu, also has a very high neutron multiplicity in the epithermal region). Thus all of the neutrons that went into making it can be recovered, and at one MeV or more (fissioning actinides general emerge at 1 - 2 MeV before being thermalized by a moderator).
This makes americium a very attractive fuel to my mind. My son's girlfriend, also a nuclear engineering Ph.D. student, reports that americium is her favorite element, which is just one of the reasons I like her very much. I have a cool idea about an americium fueled high temperature reactor, and if I can't get my son to listen to it, perhaps she will.
It's certainly available now in multiton quantities, albeit in dilute solid solutions. I think it should be readily concentrated in fluoride volatility reprocessing schemes.