Science
Related: About this forumI'll be damned if I can find out what the "syn gas power constant" is.
Generally, when I come across a term used, often utilized knowingly in some esoteric field, with which I am unfamiliar but in some context of interest, I try to find out what the term means.
I came across this paper yesterday: Impacts of Interaction between Active Components on Catalyst Deactivation over KFe/ZSM-5 Bifunctional Catalyst Di Xu, Haifeng Fan, Kaidi Liu, Guoqiang Hou, Chuan Qin, Yanfei Xu, Rui Li, Junhu Wang, and Mingyue Ding ACS Sustainable Chemistry & Engineering 2023 11 (28), 10441-10452.
This concerns an energy process that I actually oppose, coal gasification by steam reforming to produce hydrogen - a very, very, very dirty fuel - and carbon dioxide, to make what are normally petroleum based fuels, such as gasoline, jet fuel, diesel fuel, etc. This process, which was industrialized in Nazi Germany, in Apartheid era South Africa, and was an element of Jimmy Carter's energy program although the industrialization in the US has (thankfully) remained limited but for a few specialized commodity plants, is known at the Fisher-Tropsch (FT) process.
(I favor the hydrogenation of carbon dioxide to methanol and ultimately the wonder fuel DME, but only in the case where the primary energy source of hydrogen is nuclear energy using, ideally, thermochemical processes, and wet or dry reformation of wastes.)
I am interested in general in bifunctional catalysts, not for FT purposes, but because these types of catalysts have been explored for the direct hydrogenation of carbon dioxide to DME (dimethyl ether) without the production of a methanol intermediate.
So I scanned the paper and came across this sentence:
I had never heard of the Anderson-Schulz-Flory distribution, so I referred to reference 18, which is this one, dated from 73 years ago:
Composition of Synthetic Liquid Fuels. I. Product Distribution and Analysis of C5—C8 Paraffin Isomers from Cobalt Catalyst1 R. A. Friedel and R. B. Anderson Journal of the American Chemical Society 1950 72 (3), 1212-1215
It has the following text, here presented as a graphic object:
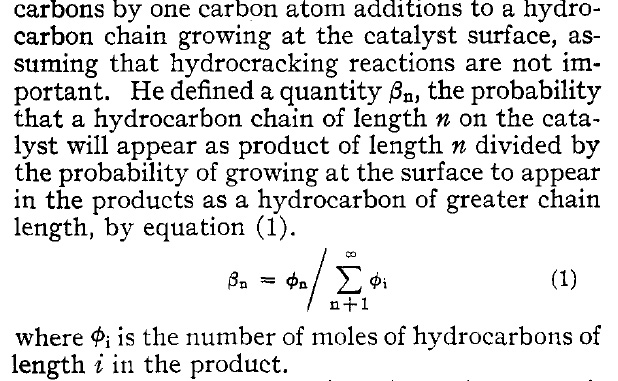
This may or may not define the modern term "syn gas power constant," which comes up flowing around this idea, as described in this paper:
Selectivity of the Fischer–Tropsch process: deviations from single alpha product distribution explained by gradients in process conditions Katrina D. Kruit David Vervloet Freek Kapteijn and J. Ruud van Ommen, Catal. Sci. Technol., 2013,3, 2210-2213
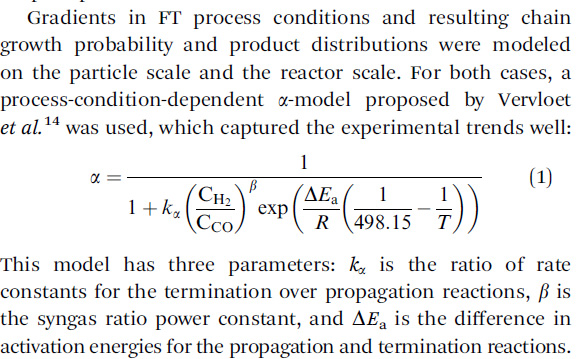
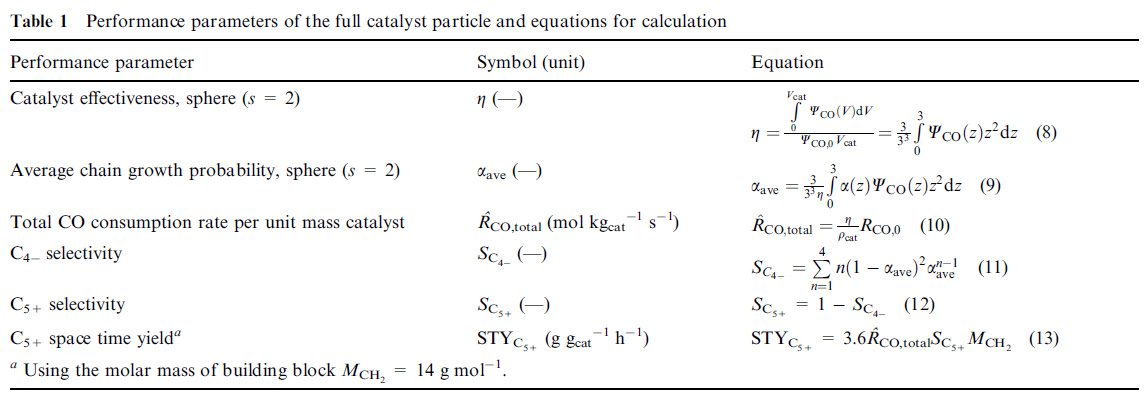
I suspect that the "syn gas power constant" is actually involved with equation 9 in this table from this paper, the "Vervloet" paper (same research group):
Fischer–Tropsch reaction–diffusion in a cobalt catalyst particle: aspects of activity and selectivity for a variable chain growth probability David Vervloet, Freek Kapteijn, John Nijenhuis and J. Ruud van Ommen Catal. Sci. Technol., 2012, 2, 1221-1233
The reality is I'm never going to want to use this "syn gas power constant" and I can't believe, with my life running out, that I spent time wondering all about it, since I rather despise the entire idea of coal based hydrogen and/or coal based syn fuels.
When I waste time this way, I feel disturbed about myself.
The FT idea sucks, and in the time of extreme climate change we are now clearly entering, it's an idea that should be abhorrent, although this FT concept is related to the awful marketing scheme represented by marketing fossil fuels as "green hydrogen."
You can read all about it in the wildland industrialization forum.
Happy Friday.
LoisB
(12,172 posts)captain queeg
(11,780 posts)Most all this discussion was over my head but it did raise a couple thoughts. I know the Nazis used coal to produce gasoline (more or less) but I’d read the gasoline produced this way was inferior and reduced its efficiency enough to hurt the performance of their fighter planes. So what was that net effect? Poorer octane?
Anyways I’m no chemist for sure. I also noticed they talked about hydro cracking, something I don’t understand but I worked one summer at an oil refinery and one of our jobs was replacing the refractory in a cracking vessel. I can’t remember if it was for hydro-cracking or cat-cracking. I’d heard both of those terms but I think they were different processes. At any rate it was a nasty job. A half dozen of us chipping off the old refractory inside a steel vessel with air chisels. I’m sure I damaged my hearing.
NNadir
(37,074 posts)It is easily liquefied, having a boiling point of around -24°C and a critical temperature of around 128°C, above the boiling point of water.
This means like propane, or LPG, it is easily liquified and stored as a liquid, and like propane, can act as a refrigerant.
Unlike propane and LPG, it is not a climate forcing gas; it's atmospheric half-life is about 5 days.
Because it lacks a carbon carbon bond, when combusted, it forms almost no particulates, although it is mildly subject, as all carbon based fuels are, to Boudouard reaction type carbon formation when burned with insufficient oxygen. The Boudouard reaction accounts for the carbon deposited in refinery reactors that on which you you worked: 2CO = CO2 + C
Because of this DME has a low pollution potential. It is easily removed from water in spills simply by aeration, it is essentially soluble in water and thus forms no harmful toxic slicks. It is generally nontoxic but exhibits a mild anesthetic effect similar to, but weaker than that by its ethyl analogue, ethyl ether. It has replaced CFC's in hairspray cans, which is its current main use.
It is well known that diesel engines can run on DME; Volvo is pushing for this idea. Thus it can displace petroleum fuels as well as natural gas, LPG, propane, etc. It will also work in spark engines. The modifications to internal combustion engines are relatively simple, involving compatible seals, carburetion adjustments, and fuel tank changes.
A diesel engine operating on DME, formed by using nuclear primary energy for a captive hydrogen source, would be less polluting than electric cars, diesels, hybrids, etc, etc, etc, and far less stupid dangerous and wasteful than the idiotic hydrogen hydra that has refused to die, cropping up big time every ten years or so, even after half a century of very, very, very, very stupid and dangerous hype.
It's cetane number is higher than diesel fuel, but its volumetric energy density is lower.
It would be entirely climate neutral, and in fact, would create a market for carbon dioxide as an industrial precursor.
It can drop in, without any major changes, to all the dangerous natural gas pipelines that lace the planet, now driving climate change. No major infrastructure change is required.
DME has not achieved the status it should because the world is conservative; we believe that everything should be done the same way it has always been done.
The DME Association is here: International DME Association.
The physical properties of DME are described here: NIST DME
Thanks for asking.