Science
Related: About this forumA high entropy alloy with exceptional fracture toughness at extreme cryogenic temperatures, 20K.
I was directed to this paper by a news link on my email feed, this one: Toughest Known Material on Earth Discovered
The full paper to which the news item refers is here: Exceptional fracture toughness of CrCoNi-based medium- and high-entropy alloys at 20 kelvin, Dong Liu, Qin Yu, Saurabh Kabra, Ming Jiang, Paul Forna-Kreutzer, Ruopeng Zhang, Madelyn Payne, Flynn Walsh, Bernd Gludovatz, Mark Asta, Andrew M. Minor, Easo P. George, Robert O. Ritchie, Science 378, 6623, 978-983 (2022).
From the introduction to the full paper:
One prominent group of such materials are the single-phase, face-centered cubic (fcc), equiatomic alloys based on the CrCoNi system. Among these, the equiatomic CrMnFeCoNi alloy is the most characterized of all HEAs (1, 6–11). It came to prominence because its room-temperature strength and ductility can be substantially enhanced at liquid nitrogen temperature (8, 9) without compromising toughness (10). Moreover, its crack-initiation fracture toughness, KJIc, remained at roughly 220 MPa·m½ over the temperature range 293 to 77 K, with a crack-growth toughness, Kss, of >300 MPa·m½ (after 2.25 mm of crack extension) (10). More recent experiments on this HEA showed a similar increase in strength and toughness (the latter measured in terms of the absorbed deviatoric strain energy) when the strain rate was increased from 10−3 s−1 (quasistatic compression) up to extremely high rates of 6 × 105 s−1 (dynamic shear) (11).
There have been several derivatives of the CrMnFeCoNi alloy (12–14), most notably the single-phase equiatomic CrCoNi medium-entropy alloy (MEA), which displays even better properties. At 77 K, this MEA was found to have a KJIc of 273 MPa·m½ and a Kss exceeding 400 MPa·m½ (15). Although strength and toughness are often mutually exclusive properties (16), the CrCoNi alloy exhibits exceptionally high damage tolerance, with fracture toughness values among the largest ever reported. Such CrCoNi-based multiple principal element alloys are clearly strong candidate materials for potential applications in extreme environments, such as at very high strain rates and cryogenic temperatures...
Much of this paper is in the language of materials science, which I only speak in a Pidgeon form; my son is a materials scientist.
However I found this graphic to be most interesting:
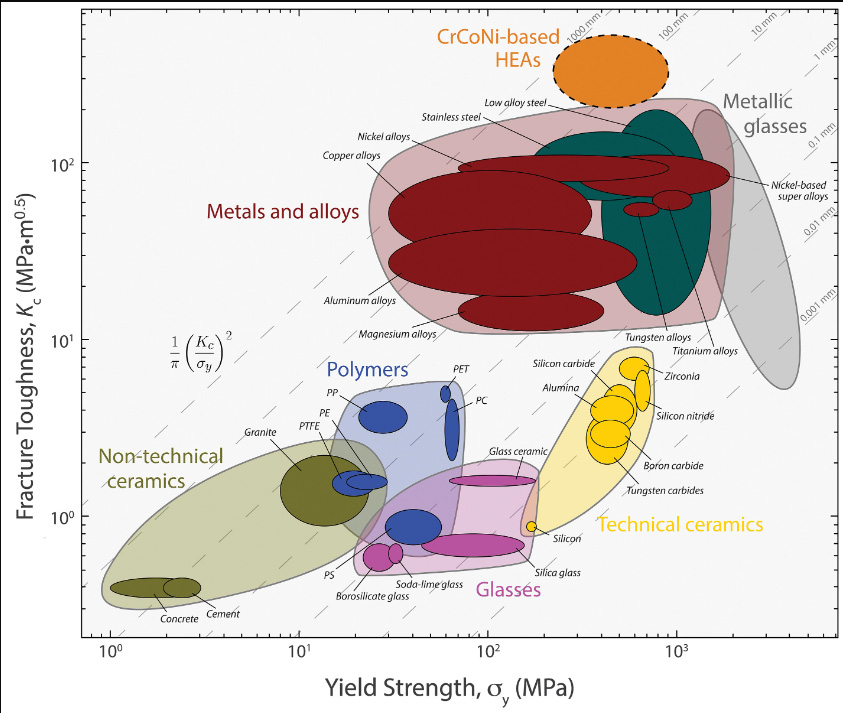
The caption:
Note the exceptional fracture toughness of the CrCoNi-based medium- and high-entropy alloys, which appear to be the highest on record. (The toughness results contained in this figure for materials other than the CrCoNi-based alloys were measured at ambient temperatures.) PC, polycarbonate; PE, polyethylene; PET, polyethylene terephthalate; PP, polypropylene; PS, polystyrene; PTFE, polytetrafluoroethylene.
Missing in this figure are the remarkable MAX phases, but it is illustrative.
Very cool...
lostnfound
(16,639 posts)Told my son it was an easy class. It was when I took it 35 years ago. Then it was about metals.. alloys, annealing, heat treating, and the basics of ceramics, not much more.
Now? It’s like 3 or 4 classes rolled into one. Their content about metals is indecipherable 9fro example now including the consequences of different types of defects at an atomic level in different types of crystal structures having effects on a whole range of material properties). I took 3 separate classes in polymers as a specialty, but that is now a significant portion of the materials class. I enjoyed fracture mechanics as a separate class, and that’s now part of this same class.
But that chart you posted is very cool and there’s a materials book that is chock full of them. They didn’t have ANYTHING like that in the 80s.
Physics seems pretty similar except that in the 80s we learned that light acted like a wave and a stream of particles, but now they have the kids calculating how 3-d oscillations of electrons (which can result from movements of electron-nucleus bonds, electron-electron interactions between atoms, or as a result of incident radiation) generate radiative electrical AND magnetic fields that actually is a wave of light… seems to me they’ve figured out how to explain it and stuffed it into sophomore physics. Now they also have to do video presentations instead of written lab reports (that’s pretty cool) and they have to learn some prorgramming.
NNadir
(34,662 posts)...Engineering.
He came to me in high school and told me that's what he wanted to be, a Materials Scientist, because it would combine physics, chemistry and math. I had only a vague understanding of it as an independent discipline. (I'm a chemist; mostly focused on bioorganic chemistry.)
I have a far greater appreciation of Materials Science now.
From what I can tell, Materials Science Engineering programs are still very small at most Universities, which is a good thing for students in the discipline, but they need to be far bigger to get humanity through the crisis in which it finds itself, particularly with respect to the environment.
Trust me, if you go beyond electrons and their orbitals, and add neutrons and recoiling nuclei to the equation, the discipline is very profound. One can, I'm sure, spend a lifetime and not scratch the surface.
I'll be giving a lecture this month that basically is about the complex metallurgy of actinide metals (where f orbitals play a role in bonding). It's not my specialty by any stretch of the imagination, but in putting the lecture together, I've developed a far deeper understanding of what my son is working through. I hope he enjoys and approves of the lecture.