Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
Editorials & Other Articles
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Environment & Energy
Related: About this forumThe Mobilization of Geogenic Uranium From Permafrost Melting.
The paper I'll discuss briefly in this post is this one:
Uranium Speciation and Mobilization in Thawing Permafrost Elliott K. Skierszkan, Valerie A. Schoepfer, Matthew Fellwock, and Matthew B. J. Lindsay Environmental Science & Technology 2024 58 (38), 17058-17069
"Geogenic uranium" is of course, naturally occurring uranium which includes of course, uranium ores. The melting permafrost discussed in this paper is found in Canada and Alaska. It is effectively impossible to deplete all the uranium on the planet; it has always been here, always will be. This is a case where uranium in natural formations is mobilized so as to show up in water.
From the introductory text:
Uranium (U) is a globally pervasive geogenic toxin with no known biological function. (1−3) The World Health Organization and the United States Environmental Protection Agency set a guideline of 30 micrograms L-1 for drinking-water quality. (4,5) Canadian guidelines are 20 micrograms L-1 for drinking water, and 15 micrograms L-1 for the protection of aquatic life. (6,7) Dissolved U concentrations in groundwater and surface water are generally less than 5 micrograms L-1, but can be 100–1000’s of times higher in regions impacted by geogenic or anthropogenic contamination. (1,3) Uranium mobility is closely tied to its oxidation state: U(VI) prevails under oxidizing conditions and has relatively higher mobility than U(IV), which forms poorly soluble phases. (8)
Organic-rich soils (ORS) are globally important U sinks, concentrating it to levels of ecotoxicological concern and economic interest. (9−15) While the average upper Earth crustal abundance is 2.7 micrograms-1 U, organic-rich peatlands downgradient of uraniferous groundwater flowpaths can accumulate Greater than 5000 micrograms g-1U. (16) This enrichment involves a combination of redox reactions and pH-dependent surface and aqueous complexation reactions that sequester U onto organic matter. (8,14−19) Reducing conditions that are characteristic of water-saturated ORS can favor U attenuation by forming insoluble noncrystalline U(IV) monomers and uraninite. (8,14,17,20,21) Several recent studies have also documented U(VI) enrichment in ORS. (14−16,18) Uranium in both its prevailing oxidation states adsorbs onto organic functional groups, which outcompete inorganic sorbents such as Fe-(oxyhydr)oxides and clays for U binding in ORS. (14,15,17,18) Uranium(VI) adsorption is typically greatest in the pH range of approximately 5 to 8, but is inhibited by U aqueous complexation with organic and inorganic ligands. (14,22) In most natural waters, dissolved organic matter (DOM) and calcium-uranyl-carbonate complexes [e.g., Ca(UO2)(CO3)3-2 Ca2(UO2)(CO3)3-2 dominate aqueous U(VI) speciation. The balance between these species is a function of pH and ligand availability (e.g., DOM, Ca2+, CO32-), which also influence electrostatic attraction of U toward adsorption sites. (22−27) In addition to inhibiting U(VI) adsorption, these aqueous complexes can enhance U mobility by inhibiting U(VI) reduction kinetics. (14,22,28,29)
Permafrost soils are estimated to contain half of the global soil carbon (C) reservoir, and are thawing at an accelerated rate because of postindustrial global warming. (30,31) Permafrost thaw is accompanied by biodegradation of previously frozen organic matter, activation of flowpaths, and increased mineral-microbe interactions. (32,33) These processes collectively drive dynamic changes in the key variables governing metal mobility [e.g., pH, oxidation–reduction potential (EH), DOM, soil organic C, and major-ion chemistry]. Recent field observations indicate that shifting hydrological conditions and biogeochemical reactions resulting from permafrost thaw mobilize metal(loid)s such as arsenic, (34) iron, (35,36) mercury, (37,38) and selenium. (39,40) To our knowledge, no studies have yet examined U speciation, abundance, or mobility in thawing permafrost soils, despite its occurrence in subpermafrost groundwater (41) and its tendency to accumulate in ORS─which are ubiquitous in (sub)arctic soils. Uranium mobilization during thaw of permafrost organic matter therefore constitutes an unknown risk to water resources in cold regions. (41,42)
In the present study, we address this knowledge gap with an analysis of the chemical speciation and abundance of U in permafrost soils and thawed porewaters collected from a subarctic study area of thawing discontinuous permafrost...
Organic-rich soils (ORS) are globally important U sinks, concentrating it to levels of ecotoxicological concern and economic interest. (9−15) While the average upper Earth crustal abundance is 2.7 micrograms-1 U, organic-rich peatlands downgradient of uraniferous groundwater flowpaths can accumulate Greater than 5000 micrograms g-1U. (16) This enrichment involves a combination of redox reactions and pH-dependent surface and aqueous complexation reactions that sequester U onto organic matter. (8,14−19) Reducing conditions that are characteristic of water-saturated ORS can favor U attenuation by forming insoluble noncrystalline U(IV) monomers and uraninite. (8,14,17,20,21) Several recent studies have also documented U(VI) enrichment in ORS. (14−16,18) Uranium in both its prevailing oxidation states adsorbs onto organic functional groups, which outcompete inorganic sorbents such as Fe-(oxyhydr)oxides and clays for U binding in ORS. (14,15,17,18) Uranium(VI) adsorption is typically greatest in the pH range of approximately 5 to 8, but is inhibited by U aqueous complexation with organic and inorganic ligands. (14,22) In most natural waters, dissolved organic matter (DOM) and calcium-uranyl-carbonate complexes [e.g., Ca(UO2)(CO3)3-2 Ca2(UO2)(CO3)3-2 dominate aqueous U(VI) speciation. The balance between these species is a function of pH and ligand availability (e.g., DOM, Ca2+, CO32-), which also influence electrostatic attraction of U toward adsorption sites. (22−27) In addition to inhibiting U(VI) adsorption, these aqueous complexes can enhance U mobility by inhibiting U(VI) reduction kinetics. (14,22,28,29)
Permafrost soils are estimated to contain half of the global soil carbon (C) reservoir, and are thawing at an accelerated rate because of postindustrial global warming. (30,31) Permafrost thaw is accompanied by biodegradation of previously frozen organic matter, activation of flowpaths, and increased mineral-microbe interactions. (32,33) These processes collectively drive dynamic changes in the key variables governing metal mobility [e.g., pH, oxidation–reduction potential (EH), DOM, soil organic C, and major-ion chemistry]. Recent field observations indicate that shifting hydrological conditions and biogeochemical reactions resulting from permafrost thaw mobilize metal(loid)s such as arsenic, (34) iron, (35,36) mercury, (37,38) and selenium. (39,40) To our knowledge, no studies have yet examined U speciation, abundance, or mobility in thawing permafrost soils, despite its occurrence in subpermafrost groundwater (41) and its tendency to accumulate in ORS─which are ubiquitous in (sub)arctic soils. Uranium mobilization during thaw of permafrost organic matter therefore constitutes an unknown risk to water resources in cold regions. (41,42)
In the present study, we address this knowledge gap with an analysis of the chemical speciation and abundance of U in permafrost soils and thawed porewaters collected from a subarctic study area of thawing discontinuous permafrost...
Some figures from the text:
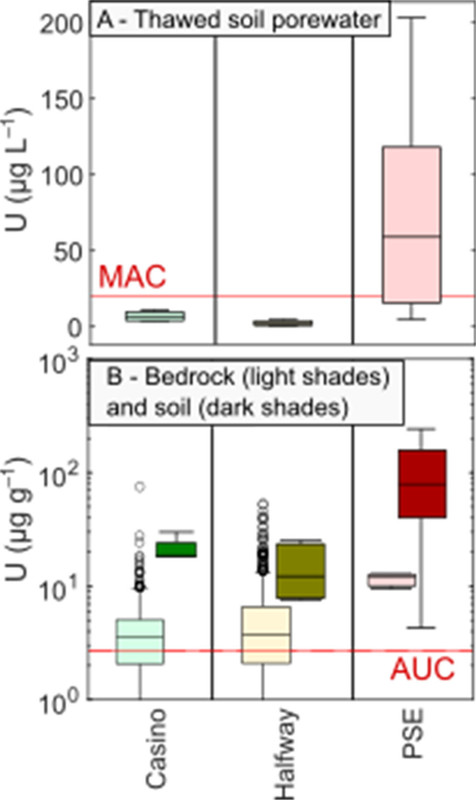
The caption:
Figure 1. Concentrations of U in (A) soil porewater; and (B) bedrock (lighter shades) and soil (darker shades), grouped by catchment. MAC = maximum allowable U concentration in Canadian drinking water; (64) AUC = average upper Earth crustal U abundance. (48) Bedrock data from Skierszkan et al. (42) and Yukon Geological Survey. (65)
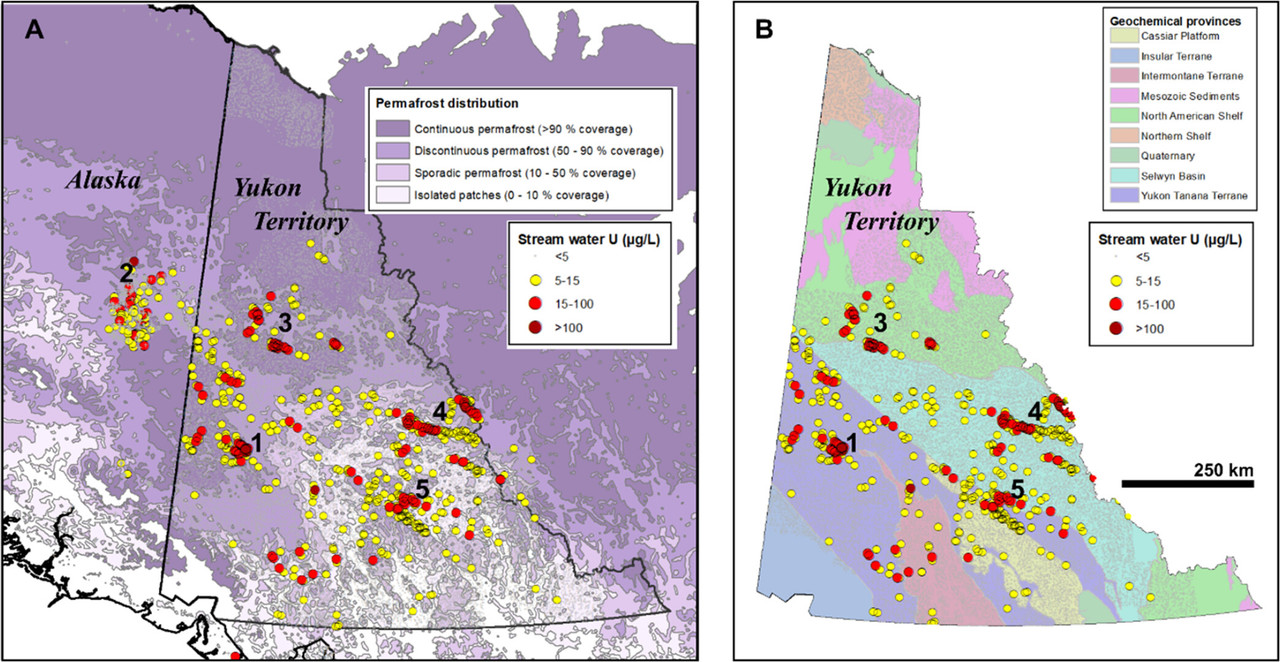
The caption:
Figure 4. Compilation of dissolved U in ∼34,000 stream water samples over western North American subarctic from Canadian (89) and American (90) federal data sets. Panel (A) shows U concentrations over the regional permafrost distribution (from Obu et al. (91)) Panel (B) shows U concentrations over various Geochemical Provinces of Yukon. (92) Numbers delineate clusters of U-rich streams over various geological terranes including: (1) and (2) Cretaceous granitic and Paleozoic metamorphic rocks of the Yukon-Tanana Terrane; (3) and (4) interbedded Paleozoic marine siliciclastic and carbonate rocks; and (5) Paleozoic marine black shales and limestones intruded by Cretaceous granites.
The paper's conclusion:
Our results reveal that permafrost ORS can be enriched in U by 10–100 s of times relative to local bedrock. This U pool is dominated by its mobile hexavalent state and is complexed onto soil organic matter. Incipient thaw of this material can produce porewater U concentrations that exceed safe thresholds (e.g., for drinking water and for the protection of aquatic life), especially downgrading of U-rich bedrock. Thermokarst processes can also export this material to surface water bodies. The fate of U after permafrost thaw is uncertain as it depends on complex and coupled interactions between fluid transport, microbial activity, and transient changes in groundwater pH, EH, major-ion chemistry, and DOM composition. The uncertain outcomes of these processes on U mobility, bioavailability, and toxicity warrant further investigation, particularly given the occurrence of U-rich geological terranes and aquifers in permafrost-dominated subarctic regions worldwide (e.g., Figure 4; Alaska, (93) Yukon, (41) Northern Canada’s U-rich Athabasca Basin, (94) Scandinavia (3,95)).
Note: The words "climate change" appear in the original text; I prefer to use the term "extreme global heating" which is a more accurate description of the current reality. I have also changed some symbols not supported by the DU editor with their equivalence in words.
I have argued that in a case of a switch from thermal neutron spectrum nuclear reactors (which dominate the world inventory of nuclear reactors) to fast neutron spectrum nuclear reactors (for which there is only a relatively small fleet), it may not be necessary to mine uranium at all for centuries, in particular in the case where it is coupled with the thorium that is now dumped as a side product of lanthanide (rare earth) mining for wind turbines and other magnetic devices. The incorporation of thorium into a class of reactors known as heavy water reactors, the most famous cases being Canada's CANDU reactor, can also function as effective breeder reactors, albeit with somewhat longer doubling times than plutonium fueled fast reactors. In this case the uranium already mined, much of it so called "depleted uranium" could supply all the world's energy demands for centuries to come without the operation of a single mine of any type, no oil wells, no fracking for gas, no coal mines. (We might also be able to restore free flowing rivers.)
This said, a great deal of research has gone into the recovery of uranium from seawater, which contains just shy of 5 billion tons of uranium, as part of a geological natural uranium cycle between the Earth's mantle, crusts, and oceans. These general involve certain classes of resins. The same resins might well be utilized to remove naturally mobilized uranium as well as uranium mobilized by mining and processing, with the resins (generally functionalized with aldoximes, although other approaches are known), with the result that the resins would become uranium ores. At current uranium prices, this is not really an economically viable approach, but with subsidies to clean up natural and anthropogenic uranium contamination, it may be viable both the extend uranium resources and to provide cleaner water.
I trust you will have a pleasant weekend.